Organovo Holdings, Inc., a development-stage company, focuses on developing and commercializing functional human tissues that could be employed in drug discovery and development, biological research, and as therapeutic implants for the treatment of damaged or degenerating tissues and organs. The company is developing a suite of standardized and three-dimensional human tissues for the preclinical assessment of drug effects, including applications in predictive toxicology, absorption, distribution, metabolism, excretion, and drug metabolism and pharmacokinetics; customized human tissues as living, dynamic models of human biology or disease, for use in drug discovery and development; and three-dimensional human tissues for clinical applications, such as blood vessels for bypass grafting and nerve grafts for nerve damage repair, as well as functional tissue patches for the repair or replacement of damaged tissues and organs. Organovo Holdings, Inc. has collaboration agreements with United Therapeutics Corporation; Knight Cancer Institute at Oregon Health & Science University; Michael J. Fox Foundation; Hoffman La Roche; Janssen Pharmaceuticals; and L'Oreal. The company was founded in 2007 and is headquartered in San Diego, California.
Analise Tecnica:

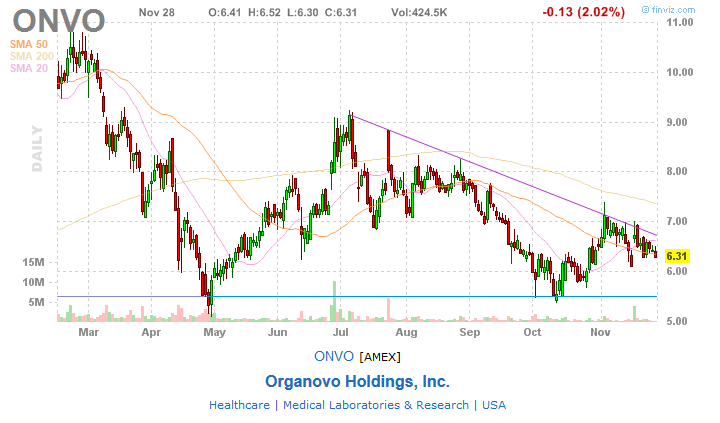
A cotação encontra-se numa tendência de subida a corrigir nos últimos dias á média movel, acima de um forte suporte e com uma linha de resistência descendente que poderá travar a subida da cotação.
Análise Fundamental:
Não existem dados suficientes para aplicar o modelo económico futuro.
Apresentou resultados a dia 7 de Novembro.
Form 10-Q for ORGANOVO HOLDINGS, INC.
7-Nov-2014
Quarterly Report
Item 2. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following management's discussion and analysis should be read in conjunction with the Company's historical consolidated financial statements and the related notes thereto included in our Annual Report on Form 10-K for the fiscal year ended March 31, 2014. The management's discussion and analysis contains forward-looking statements, such as statements of our plans, objectives, expectations and intentions. Any statements that are not statements of historical fact are forward-looking statements. When used, the words "believe," "plan," "intend," "anticipate," "target," "estimate," "expect" and the like, and/or future tense or conditional constructions such as "will," "may," "could," "should,", or similar expressions, identify certain of these forward-looking statements. These forward-looking statements speak only as of the date of this Quarterly Report on Form 10-Q and are subject to risks and uncertainties, including those described in "Item 1A-Risk Factors" of this Quarterly Report on Form 10-Q and our Annual Report on Form 10-K for the fiscal year ended March 31, 2014 that could cause our actual results or events to differ materially from those expressed or implied by such forward-looking statements. Except to the limited extent required by applicable law, the Company does not undertake any obligation to update forward-looking statements to reflect events or circumstances occurring after the date of this Quarterly Report.
Basis of Presentation
References in this section to "Organovo Holdings, Inc.," "Organovo Holdings," "we," "us," "our," "the Company" and "our Company" refer to Organovo Holdings, Inc. and its consolidated subsidiary Organovo, Inc.
The condensed consolidated financial statements included in this Form 10-Q have been prepared in accordance with the SEC instructions to Quarterly Reports on Form 10-Q. Accordingly, the condensed consolidated financial statements presented elsewhere in this Form 10-Q and discussed below are unaudited and do not contain all the information required by U.S. generally accepted accounting principles ("GAAP") to be included in a full set of financial statements. The audited financial statements for the year ended March 31, 2014, filed with the SEC on Form 10-K on June 10, 2014 include a summary of our significant accounting policies and should be read in conjunction with this Form 10-Q. In the opinion of management, all material adjustments necessary to present fairly the results of operations for such periods have been included in this Form 10-Q. All such adjustments are of a normal recurring nature. The results of operations for interim periods are not necessarily indicative of the results of operations for the entire year.
Overview
Organovo, Inc. was founded in Delaware in April 2007. Activities since Organovo, Inc.'s inception through September 30, 2014 were devoted primarily to developing functional three-dimensional (3D) human tissues that can be employed in drug discovery and development, biological research, and as therapeutic implants for the treatment of damaged or degenerating tissues and organs. As of September 30, 2014, Organovo, Inc. has devoted substantially all of its efforts to product development, raising capital and building infrastructure. Organovo, Inc. has not, as of that date, realized significant revenues from its planned principal operations.
On February 8, 2012, Organovo, Inc., a privately held Delaware corporation, merged with and into Organovo Acquisition Corp., a wholly-owned subsidiary of the Company, with Organovo, Inc. surviving the merger as a wholly-owned subsidiary of the Company (the "Merger"). As a result of the Merger, the Company acquired the business of Organovo, Inc., and will continue the existing business operations of Organovo, Inc.
Critical Accounting Policies, Estimates, and Judgments
Our financial statements are prepared in accordance with accounting principles that are generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. We continually evaluate our estimates and judgments, the most critical of which are those related to revenue recognition, valuation of long-lived assets and warrant liability, stock-based compensation and the timing of the achievement of collaboration milestones. We base our estimates and judgments on historical experience and other factors that we believe to be reasonable under the circumstances. Materially different results can occur as circumstances change and additional information becomes known. Besides the estimates identified above that are considered critical, we make many other accounting estimates in preparing our financial statements and related disclosures. All estimates, whether or not deemed critical, affect reported amounts of assets, liabilities, revenues and expenses, as well as disclosures of contingent assets and liabilities. These estimates and judgments are also based on historical experience and other factors that are believed to be reasonable under the circumstances. Materially different results can occur as circumstances change and additional information becomes known, even for estimates and judgments that are not deemed critical.
Table of Contents
For further information, refer to the Company's audited financial statements and notes thereto included in the Annual Report on Form 10-K for the year ended March 31, 2014, filed with the Securities and Exchange Commission (the "SEC") on June 10, 2014.
Results of Operations
Comparison of the three months ended September 30, 2014 and 2013
Revenues
For the three months ended September 30, 2014, total revenues of $50,000 were $27,000 or 117% above the $23,000 in revenues for the same period in 2013. For both periods, the majority of revenues were derived from collaborative research agreements. POSITIVE
Operating Expenses
Overview
Operating expenses increased approximately $3.4 million, or 61%, from approximately $5.6 million for the three months ended September 30, 2013 to $9.0 million for the three months ended September 30, 2014. Of this increase, $1.8 million relates to increased selling, general and administrative expense while the other $1.6 million relates to increased investment in research and development. These increases can be attributed to the Company's continued implementation of its business plan, including hiring additional staff to support research and development initiatives, incremental investments associated with strategic growth and commercialization project initiatives, expenses related to operating as a publicly traded corporation, expansion of its facility, and increased stock compensation expense relative to employees and certain consulting services.
Research and Development Expenses
Research and development expenses increased nearly 100% from approximately $1.6 million for the three months ended September 30, 2013 to $3.2 million for the three months ended September 30, 2014, as the Company increased its research staff to support its obligations under certain collaborative research agreements and to expand its product development efforts in preparation for research-derived revenues. Full-time research and development staffing increased from twenty-four full-time employees as of September 30, 2013 to forty-four full-time employees as of September 30, 2014. In addition to an increase in staffing expense of approximately $0.5 million and an increase in stock-based compensation of $0.2 million resulting from increased headcount and increasing stock prices from September 30, 2013 to September 30, 2014, the Company increased its spending on recruiting, lab equipment and supplies in proportion to its increased research activities. In addition, the Company has continued to invest additional resources to advance its bioprinting technology during the period.
General and Administrative Expenses
For the three months ended September 30, 2014, general and administrative expenses were approximately $5.8 million, an increase of $1.8 million, or 44%, over expenses in the same period of 2013 of approximately $4.0 million. Stock-based compensation increased $0.6 million due to additional grants and increasing stock prices from September 30, 2013 to September 30, 2014. Staffing expense increased $0.4 million due to an increase in administrative headcount from twelve full-time employees to sixteen full-time employees to provide strategic infrastructure in developing collaborative relationships and preparation for commercialization of research-derived product introductions. In addition, the Company incurred additional expenses for investor outreach initiatives and consulting activities in the three months ended September 30, 2014 as compared to the three months ended September 30, 2013.
Other Income (Expense)
Other income was less than $0.1 million for the three months ended September 30, 2014 and consisted primarily of a gain related to the revaluation of warrant derivative liabilities. This gain was caused by a declining stock price during the quarter that decreased the value of the derivative liability. For the three months ended September 30, 2013, other expense consisted primarily of a $4.8 million loss related to the revaluation of the warrant derivative liability due to rising stock prices during the period that caused an increase in the value of the derivative liability. In addition, the majority of the underlying warrants to which the derivative relates were exercised or converted to equity instruments during fiscal 2014, significantly lessening the impact of subsequent changes in our stock price.
NEGATIVE
Table of Contents
Comparison of the six months ended September 30, 2014 and 2013
Revenues
For the six months ended September 30, 2014, total revenues of $0.1 million were consistent with the $0.1 million in revenues for the same period in 2013. For both periods, the majority of revenues were derived from collaborative research agreements.
Operating Expenses
Overview
Operating expenses increased approximately $6.0 million, or 64%, from approximately $9.5 million for the six months ended September 30, 2013 to $15.5 million for the six months ended September 30, 2014. Of this increase, $3.1 million is related to increased selling, general and administrative expense while the other $2.9 million relates to increased investment in research and development. These increases are attributed to the Company's continued implementation of its business plan, including hiring additional staff to support research and development initiatives, incremental investments associated with strategic growth and commercialization project initiatives, expenses related to operating as a publicly traded corporation, expansion of its facility, and increased stock compensation expense relative to employees and certain consulting services.
Research and Development Expenses
Research and development expenses increased by approximately $2.9 million, or 95%, from approximately $3.1 million for the six months ended September 30, 2013 to approximately $6.0 million for the six months ended September 30, 2014, as the Company increased its research staff to support its obligations under certain collaborative research agreements and to expand its product development efforts in preparation for research-derived revenues. Full-time research and development staffing increased from twenty-four full-time employees as of September 30, 2013 to forty-four full-time employees as of September 30, 2014. In addition to increases in staffing expense of approximately $0.9 million and an increase in stock-based compensation of $0.4 million resulting from increased headcount and increasing stock prices from September 30, 2013 to September 30, 2014, the Company increased its spending on recruiting, lab equipment, supplies and contracted services in proportion to its increased research activities. In addition, the Company has continued to invest additional resources to advance its bioprinting technology during the period.
General and Administrative Expenses
For the six months ended September 30, 2014, general and administrative expenses were approximately $9.5 million, an increase of $3.1 million, or 49%, over expenses in the same period of 2013 of approximately $6.4 million. Stock-based compensation increased $1.1 million due to additional grants and increasing stock prices from September 30, 2013 to September 30, 2014. Staffing expense increased $0.7 million due to an increase in administrative headcount from twelve full-time employees to sixteen full-time employees to provide strategic infrastructure in developing collaborative relationships and preparation for commercialization of research-derived product introductions. In addition, the Company incurred additional expenses for investor outreach initiatives, consulting and legal activities in the six months ended September 30, 2014 as compared to the six months ended September 30, 2013.
Other Income (Expense)
Other income was less than $0.1 million for the six months ended September 30, 2014 and consisted primarily of a gain related to the revaluation of warrant derivative liabilities. This gain was caused by a declining stock price during the period that decreased the value of the derivative liability. For the six months ended September 30, 2013, other expense consisted primarily of a $4.8 million loss related to the revaluation of the warrant derivative liability due to rising stock prices during the period that caused an increase in the value of the derivative liability. In addition, the majority of the underlying warrants to which the derivative relates were exercised or converted to equity instruments during fiscal 2014, significantly lessening the impact of subsequent changes in our stock price.
Financial Condition, Liquidity and Capital Resources
Since its inception, the Company has primarily devoted its efforts to research and development, business planning, raising capital, recruiting management and technical staff, and acquiring operating assets. The Company has not commenced principal operations or realized significant revenue therefrom.
Since inception, the Company incurred negative cash flows from operations. As of September 30, 2014, the Company had cash and cash equivalents of approximately $54.4 million and an accumulated deficit of $107.5 million. The Company also had negative cash flow from operations of $9.7 million during the six months ended September 30, 2014. At March 31, 2014, the Company had cash and cash equivalents of approximately $48.2 million and an accumulated deficit of $92.2 million.
Table of Contents
At September 30, 2014, the Company had total current assets of approximately $55.1 million and current liabilities of approximately $3.1 million, resulting in working capital of $52.0 million. At March 31, 2014, we had total current assets of approximately $49.2 million and current liabilities of approximately $1.9 million, resulting in working capital of $47.3 million.
Net cash used by operating activities for the six months ended September 30, 2014 was approximately $9.7 million as compared to $6.3 million used in operating activities for the six months ended September 30, 2013. This $3.4 million increase in cash usage can be attributed to a $6.0 million increase in operating expenses, partially offset by an overall increase of $2.0 million of non-cash expenses included in operations, including stock-based compensation, depreciation and amortization, as well as changes in working capital.
Net cash used in investing activities was approximately $0.6 million and $0.1 million for the six months ended September 30, 2014 and 2013, respectively. This increase can be attributed to increased capital spending as the company expands its research capabilities.
Net cash provided by financing activities decreased from $44.1 million provided during the six months ended September 30, 2013 to $16.5 million provided during the six months ended September 30, 2014. This decrease is primarily due to the inclusion of $43.4 million in net proceeds from the issuance of common stock during the six months ended September 30, 2013, as compared to $16.1 million in net proceeds from the issuance of common stock during the six months ended September 30, 2014.
We believe our cash and cash equivalents on hand as of September 30, 2014, together with amounts to be received from our collaborative research agreements and the commercialization of our products and services, should be sufficient to fund our ongoing operations as currently planned for at least the next twelve months. Through September 30, 2014, we have financed our operations primarily through the sale of convertible notes, the private placement of equity securities, the sale of common stock through public offerings, and from revenue derived from grants or collaborative research agreements.
In addition, in November 2013, the Company entered into an equity distribution agreement with an investment banking firm. Under the terms of the distribution agreement, the Company may offer and sell up to 4,000,000 shares of its common stock, from time to time, through the investment bank in "at the market" offerings, as defined by the SEC, and pursuant to the Company's effective shelf registration statement previously filed with the SEC. During the three and six months ended September 30, 2014, the Company issued 2,197,768 shares of common stock in at the market offerings under the distribution agreement with net proceeds of $16.1 million. We intend to use the net proceeds from these offerings for general corporate purposes, including research and development, the development and commercialization of our products, general administrative expenses, license or technology acquisitions, and working capital and capital expenditures. We may also use the net proceeds to repay any debts and/or invest in or acquire complementary businesses, products or technologies, although we have no current commitments or agreements with respect to any such investments or acquisitions as of the date of this Quarterly Report on Form 10-Q.
The Company will need additional capital to further fund product development and commercialization of its human tissues that can be employed in drug discovery and development, biological research, and as therapeutic implants for the treatment of damaged or degenerating tissues and organs. The Company intends to cover its future operating expenses through cash on hand, through additional financing from existing and prospective investors, from revenue derived from grants and collaborative research agreements, and revenue from the commercialization of products and services. However, we may not be successful in obtaining sufficient funding to support our operations from new or existing collaborative research agreements or the commercialization of products and services. In addition, we cannot be sure that additional financing will be available when needed or that, if available, financing will be obtained on terms favorable to us or to our stockholders. Further, we cannot assure you that we will receive 100% of the potential funding under existing grants, and we may not be successful in securing additional grants in the future.
Having insufficient funds may require us to delay, scale back, or eliminate some or all of our development programs or relinquish rights to our technology on less favorable terms than we would otherwise choose. Failure to obtain adequate financing could eventually adversely affect our ability to operate as a going concern. If we raise additional funds from the issuance of equity securities, substantial dilution to our existing stockholders would likely result. If we raise additional funds by incurring debt financing, the terms of the debt may involve significant cash payment obligations as well as covenants and specific financial ratios that may restrict our ability to operate our business.
As of September 30, 2014, the Company had 80,404,648 total issued and outstanding shares of Common Stock, and five year warrants for the opportunity to purchase an additional 869,239 shares of Common Stock at exercise prices between $0.85 and $1.00 per share and 263,870 warrants with terms between two and five years and exercise prices between $2.21 and $7.62 per share. If all warrants were exercised on a cash basis, the Company would realize approximately $2.3 million additional gross proceeds.
Table of Contents
The 2008 Equity Incentive Plan provides for the issuance of up to 896,256 shares of our outstanding Common Stock and the 2012 Equity Incentive Plan, as amended, provides for the issuance of up to 11,553,986 shares, or approximately 16% of our outstanding Common Stock, to executive officers, directors, advisory board members, employees and consultants. In aggregate, issued and outstanding common stock, shares underlying outstanding warrants, and shares reserved for the 2008 and 2012 incentive plans total 91,712,297 shares of common stock as of September 30, 2014.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements, including unrecorded derivative instruments that have or are reasonably likely to have a current or future material effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources. We have certain warrants and options outstanding but we do not expect to receive sufficient proceeds from the exercise of these instruments unless and until the underlying securities are registered, and/or all restrictions on trading, if any, are removed, and in either case the trading price of our Common Stock is significantly greater than the applicable exercise prices of the options and warrants.
Effect of Inflation and Changes in Prices
Management does not believe that inflation and changes in price will have a material effect on the Company's operations.
Recomendação:
Pelo gráfico técnico julgo que a tendência é de subida.
Analise Tecnica:

A cotação encontra-se numa tendência de subida a corrigir nos últimos dias á média movel, acima de um forte suporte e com uma linha de resistência descendente que poderá travar a subida da cotação.
Análise Fundamental:
Não existem dados suficientes para aplicar o modelo económico futuro.
Apresentou resultados a dia 7 de Novembro.
Form 10-Q for ORGANOVO HOLDINGS, INC.
7-Nov-2014
Quarterly Report
Item 2. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following management's discussion and analysis should be read in conjunction with the Company's historical consolidated financial statements and the related notes thereto included in our Annual Report on Form 10-K for the fiscal year ended March 31, 2014. The management's discussion and analysis contains forward-looking statements, such as statements of our plans, objectives, expectations and intentions. Any statements that are not statements of historical fact are forward-looking statements. When used, the words "believe," "plan," "intend," "anticipate," "target," "estimate," "expect" and the like, and/or future tense or conditional constructions such as "will," "may," "could," "should,", or similar expressions, identify certain of these forward-looking statements. These forward-looking statements speak only as of the date of this Quarterly Report on Form 10-Q and are subject to risks and uncertainties, including those described in "Item 1A-Risk Factors" of this Quarterly Report on Form 10-Q and our Annual Report on Form 10-K for the fiscal year ended March 31, 2014 that could cause our actual results or events to differ materially from those expressed or implied by such forward-looking statements. Except to the limited extent required by applicable law, the Company does not undertake any obligation to update forward-looking statements to reflect events or circumstances occurring after the date of this Quarterly Report.
Basis of Presentation
References in this section to "Organovo Holdings, Inc.," "Organovo Holdings," "we," "us," "our," "the Company" and "our Company" refer to Organovo Holdings, Inc. and its consolidated subsidiary Organovo, Inc.
The condensed consolidated financial statements included in this Form 10-Q have been prepared in accordance with the SEC instructions to Quarterly Reports on Form 10-Q. Accordingly, the condensed consolidated financial statements presented elsewhere in this Form 10-Q and discussed below are unaudited and do not contain all the information required by U.S. generally accepted accounting principles ("GAAP") to be included in a full set of financial statements. The audited financial statements for the year ended March 31, 2014, filed with the SEC on Form 10-K on June 10, 2014 include a summary of our significant accounting policies and should be read in conjunction with this Form 10-Q. In the opinion of management, all material adjustments necessary to present fairly the results of operations for such periods have been included in this Form 10-Q. All such adjustments are of a normal recurring nature. The results of operations for interim periods are not necessarily indicative of the results of operations for the entire year.
Overview
Organovo, Inc. was founded in Delaware in April 2007. Activities since Organovo, Inc.'s inception through September 30, 2014 were devoted primarily to developing functional three-dimensional (3D) human tissues that can be employed in drug discovery and development, biological research, and as therapeutic implants for the treatment of damaged or degenerating tissues and organs. As of September 30, 2014, Organovo, Inc. has devoted substantially all of its efforts to product development, raising capital and building infrastructure. Organovo, Inc. has not, as of that date, realized significant revenues from its planned principal operations.
On February 8, 2012, Organovo, Inc., a privately held Delaware corporation, merged with and into Organovo Acquisition Corp., a wholly-owned subsidiary of the Company, with Organovo, Inc. surviving the merger as a wholly-owned subsidiary of the Company (the "Merger"). As a result of the Merger, the Company acquired the business of Organovo, Inc., and will continue the existing business operations of Organovo, Inc.
Critical Accounting Policies, Estimates, and Judgments
Our financial statements are prepared in accordance with accounting principles that are generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. We continually evaluate our estimates and judgments, the most critical of which are those related to revenue recognition, valuation of long-lived assets and warrant liability, stock-based compensation and the timing of the achievement of collaboration milestones. We base our estimates and judgments on historical experience and other factors that we believe to be reasonable under the circumstances. Materially different results can occur as circumstances change and additional information becomes known. Besides the estimates identified above that are considered critical, we make many other accounting estimates in preparing our financial statements and related disclosures. All estimates, whether or not deemed critical, affect reported amounts of assets, liabilities, revenues and expenses, as well as disclosures of contingent assets and liabilities. These estimates and judgments are also based on historical experience and other factors that are believed to be reasonable under the circumstances. Materially different results can occur as circumstances change and additional information becomes known, even for estimates and judgments that are not deemed critical.
Table of Contents
For further information, refer to the Company's audited financial statements and notes thereto included in the Annual Report on Form 10-K for the year ended March 31, 2014, filed with the Securities and Exchange Commission (the "SEC") on June 10, 2014.
Results of Operations
Comparison of the three months ended September 30, 2014 and 2013
Revenues
For the three months ended September 30, 2014, total revenues of $50,000 were $27,000 or 117% above the $23,000 in revenues for the same period in 2013. For both periods, the majority of revenues were derived from collaborative research agreements. POSITIVE
Operating Expenses
Overview
Operating expenses increased approximately $3.4 million, or 61%, from approximately $5.6 million for the three months ended September 30, 2013 to $9.0 million for the three months ended September 30, 2014. Of this increase, $1.8 million relates to increased selling, general and administrative expense while the other $1.6 million relates to increased investment in research and development. These increases can be attributed to the Company's continued implementation of its business plan, including hiring additional staff to support research and development initiatives, incremental investments associated with strategic growth and commercialization project initiatives, expenses related to operating as a publicly traded corporation, expansion of its facility, and increased stock compensation expense relative to employees and certain consulting services.
Research and Development Expenses
Research and development expenses increased nearly 100% from approximately $1.6 million for the three months ended September 30, 2013 to $3.2 million for the three months ended September 30, 2014, as the Company increased its research staff to support its obligations under certain collaborative research agreements and to expand its product development efforts in preparation for research-derived revenues. Full-time research and development staffing increased from twenty-four full-time employees as of September 30, 2013 to forty-four full-time employees as of September 30, 2014. In addition to an increase in staffing expense of approximately $0.5 million and an increase in stock-based compensation of $0.2 million resulting from increased headcount and increasing stock prices from September 30, 2013 to September 30, 2014, the Company increased its spending on recruiting, lab equipment and supplies in proportion to its increased research activities. In addition, the Company has continued to invest additional resources to advance its bioprinting technology during the period.
General and Administrative Expenses
For the three months ended September 30, 2014, general and administrative expenses were approximately $5.8 million, an increase of $1.8 million, or 44%, over expenses in the same period of 2013 of approximately $4.0 million. Stock-based compensation increased $0.6 million due to additional grants and increasing stock prices from September 30, 2013 to September 30, 2014. Staffing expense increased $0.4 million due to an increase in administrative headcount from twelve full-time employees to sixteen full-time employees to provide strategic infrastructure in developing collaborative relationships and preparation for commercialization of research-derived product introductions. In addition, the Company incurred additional expenses for investor outreach initiatives and consulting activities in the three months ended September 30, 2014 as compared to the three months ended September 30, 2013.
Other Income (Expense)
Other income was less than $0.1 million for the three months ended September 30, 2014 and consisted primarily of a gain related to the revaluation of warrant derivative liabilities. This gain was caused by a declining stock price during the quarter that decreased the value of the derivative liability. For the three months ended September 30, 2013, other expense consisted primarily of a $4.8 million loss related to the revaluation of the warrant derivative liability due to rising stock prices during the period that caused an increase in the value of the derivative liability. In addition, the majority of the underlying warrants to which the derivative relates were exercised or converted to equity instruments during fiscal 2014, significantly lessening the impact of subsequent changes in our stock price.
NEGATIVE
Table of Contents
Comparison of the six months ended September 30, 2014 and 2013
Revenues
For the six months ended September 30, 2014, total revenues of $0.1 million were consistent with the $0.1 million in revenues for the same period in 2013. For both periods, the majority of revenues were derived from collaborative research agreements.
Operating Expenses
Overview
Operating expenses increased approximately $6.0 million, or 64%, from approximately $9.5 million for the six months ended September 30, 2013 to $15.5 million for the six months ended September 30, 2014. Of this increase, $3.1 million is related to increased selling, general and administrative expense while the other $2.9 million relates to increased investment in research and development. These increases are attributed to the Company's continued implementation of its business plan, including hiring additional staff to support research and development initiatives, incremental investments associated with strategic growth and commercialization project initiatives, expenses related to operating as a publicly traded corporation, expansion of its facility, and increased stock compensation expense relative to employees and certain consulting services.
Research and Development Expenses
Research and development expenses increased by approximately $2.9 million, or 95%, from approximately $3.1 million for the six months ended September 30, 2013 to approximately $6.0 million for the six months ended September 30, 2014, as the Company increased its research staff to support its obligations under certain collaborative research agreements and to expand its product development efforts in preparation for research-derived revenues. Full-time research and development staffing increased from twenty-four full-time employees as of September 30, 2013 to forty-four full-time employees as of September 30, 2014. In addition to increases in staffing expense of approximately $0.9 million and an increase in stock-based compensation of $0.4 million resulting from increased headcount and increasing stock prices from September 30, 2013 to September 30, 2014, the Company increased its spending on recruiting, lab equipment, supplies and contracted services in proportion to its increased research activities. In addition, the Company has continued to invest additional resources to advance its bioprinting technology during the period.
General and Administrative Expenses
For the six months ended September 30, 2014, general and administrative expenses were approximately $9.5 million, an increase of $3.1 million, or 49%, over expenses in the same period of 2013 of approximately $6.4 million. Stock-based compensation increased $1.1 million due to additional grants and increasing stock prices from September 30, 2013 to September 30, 2014. Staffing expense increased $0.7 million due to an increase in administrative headcount from twelve full-time employees to sixteen full-time employees to provide strategic infrastructure in developing collaborative relationships and preparation for commercialization of research-derived product introductions. In addition, the Company incurred additional expenses for investor outreach initiatives, consulting and legal activities in the six months ended September 30, 2014 as compared to the six months ended September 30, 2013.
Other Income (Expense)
Other income was less than $0.1 million for the six months ended September 30, 2014 and consisted primarily of a gain related to the revaluation of warrant derivative liabilities. This gain was caused by a declining stock price during the period that decreased the value of the derivative liability. For the six months ended September 30, 2013, other expense consisted primarily of a $4.8 million loss related to the revaluation of the warrant derivative liability due to rising stock prices during the period that caused an increase in the value of the derivative liability. In addition, the majority of the underlying warrants to which the derivative relates were exercised or converted to equity instruments during fiscal 2014, significantly lessening the impact of subsequent changes in our stock price.
Financial Condition, Liquidity and Capital Resources
Since its inception, the Company has primarily devoted its efforts to research and development, business planning, raising capital, recruiting management and technical staff, and acquiring operating assets. The Company has not commenced principal operations or realized significant revenue therefrom.
Since inception, the Company incurred negative cash flows from operations. As of September 30, 2014, the Company had cash and cash equivalents of approximately $54.4 million and an accumulated deficit of $107.5 million. The Company also had negative cash flow from operations of $9.7 million during the six months ended September 30, 2014. At March 31, 2014, the Company had cash and cash equivalents of approximately $48.2 million and an accumulated deficit of $92.2 million.
Table of Contents
At September 30, 2014, the Company had total current assets of approximately $55.1 million and current liabilities of approximately $3.1 million, resulting in working capital of $52.0 million. At March 31, 2014, we had total current assets of approximately $49.2 million and current liabilities of approximately $1.9 million, resulting in working capital of $47.3 million.
Net cash used by operating activities for the six months ended September 30, 2014 was approximately $9.7 million as compared to $6.3 million used in operating activities for the six months ended September 30, 2013. This $3.4 million increase in cash usage can be attributed to a $6.0 million increase in operating expenses, partially offset by an overall increase of $2.0 million of non-cash expenses included in operations, including stock-based compensation, depreciation and amortization, as well as changes in working capital.
Net cash used in investing activities was approximately $0.6 million and $0.1 million for the six months ended September 30, 2014 and 2013, respectively. This increase can be attributed to increased capital spending as the company expands its research capabilities.
Net cash provided by financing activities decreased from $44.1 million provided during the six months ended September 30, 2013 to $16.5 million provided during the six months ended September 30, 2014. This decrease is primarily due to the inclusion of $43.4 million in net proceeds from the issuance of common stock during the six months ended September 30, 2013, as compared to $16.1 million in net proceeds from the issuance of common stock during the six months ended September 30, 2014.
We believe our cash and cash equivalents on hand as of September 30, 2014, together with amounts to be received from our collaborative research agreements and the commercialization of our products and services, should be sufficient to fund our ongoing operations as currently planned for at least the next twelve months. Through September 30, 2014, we have financed our operations primarily through the sale of convertible notes, the private placement of equity securities, the sale of common stock through public offerings, and from revenue derived from grants or collaborative research agreements.
In addition, in November 2013, the Company entered into an equity distribution agreement with an investment banking firm. Under the terms of the distribution agreement, the Company may offer and sell up to 4,000,000 shares of its common stock, from time to time, through the investment bank in "at the market" offerings, as defined by the SEC, and pursuant to the Company's effective shelf registration statement previously filed with the SEC. During the three and six months ended September 30, 2014, the Company issued 2,197,768 shares of common stock in at the market offerings under the distribution agreement with net proceeds of $16.1 million. We intend to use the net proceeds from these offerings for general corporate purposes, including research and development, the development and commercialization of our products, general administrative expenses, license or technology acquisitions, and working capital and capital expenditures. We may also use the net proceeds to repay any debts and/or invest in or acquire complementary businesses, products or technologies, although we have no current commitments or agreements with respect to any such investments or acquisitions as of the date of this Quarterly Report on Form 10-Q.
The Company will need additional capital to further fund product development and commercialization of its human tissues that can be employed in drug discovery and development, biological research, and as therapeutic implants for the treatment of damaged or degenerating tissues and organs. The Company intends to cover its future operating expenses through cash on hand, through additional financing from existing and prospective investors, from revenue derived from grants and collaborative research agreements, and revenue from the commercialization of products and services. However, we may not be successful in obtaining sufficient funding to support our operations from new or existing collaborative research agreements or the commercialization of products and services. In addition, we cannot be sure that additional financing will be available when needed or that, if available, financing will be obtained on terms favorable to us or to our stockholders. Further, we cannot assure you that we will receive 100% of the potential funding under existing grants, and we may not be successful in securing additional grants in the future.
Having insufficient funds may require us to delay, scale back, or eliminate some or all of our development programs or relinquish rights to our technology on less favorable terms than we would otherwise choose. Failure to obtain adequate financing could eventually adversely affect our ability to operate as a going concern. If we raise additional funds from the issuance of equity securities, substantial dilution to our existing stockholders would likely result. If we raise additional funds by incurring debt financing, the terms of the debt may involve significant cash payment obligations as well as covenants and specific financial ratios that may restrict our ability to operate our business.
As of September 30, 2014, the Company had 80,404,648 total issued and outstanding shares of Common Stock, and five year warrants for the opportunity to purchase an additional 869,239 shares of Common Stock at exercise prices between $0.85 and $1.00 per share and 263,870 warrants with terms between two and five years and exercise prices between $2.21 and $7.62 per share. If all warrants were exercised on a cash basis, the Company would realize approximately $2.3 million additional gross proceeds.
Table of Contents
The 2008 Equity Incentive Plan provides for the issuance of up to 896,256 shares of our outstanding Common Stock and the 2012 Equity Incentive Plan, as amended, provides for the issuance of up to 11,553,986 shares, or approximately 16% of our outstanding Common Stock, to executive officers, directors, advisory board members, employees and consultants. In aggregate, issued and outstanding common stock, shares underlying outstanding warrants, and shares reserved for the 2008 and 2012 incentive plans total 91,712,297 shares of common stock as of September 30, 2014.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements, including unrecorded derivative instruments that have or are reasonably likely to have a current or future material effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources. We have certain warrants and options outstanding but we do not expect to receive sufficient proceeds from the exercise of these instruments unless and until the underlying securities are registered, and/or all restrictions on trading, if any, are removed, and in either case the trading price of our Common Stock is significantly greater than the applicable exercise prices of the options and warrants.
Effect of Inflation and Changes in Prices
Management does not believe that inflation and changes in price will have a material effect on the Company's operations.
Recomendação:
Pelo gráfico técnico julgo que a tendência é de subida.

 Início
Início